A porous form of graphene, the world’s thinnest and lightest nanomaterial, could help bring about the quantum leap in battery efficiency that’s needed to better harness renewable energy
The future, we’re told, will run on batteries. Fully electric vehicles will become the industry standard, running fast and far on a single charge. Our phone and laptop batteries will last for days and recharge in minutes. Our homes may even power themselves, storing energy from rooftop solar panels in lightweight and long-lasting battery packs.
One thing’s clear, though: If this battery-powered future is going to happen, we need a quantum leap in battery technology. Current lithium-ion batteries have hit a wall. For the past decade, researchers have been experimenting with new materials and novel designs to build batteries that are more powerful, last longer, and charge faster. 
This week, a team of researchers from the United States, China, and Saudi Arabia unveiled a new type of battery electrode made with “holey” graphene. In a paper published in Science, the researchers describe a porous form of graphene — the world’s thinnest and lightest nanomaterial — that overcomes some key challenges in creating next-generation batteries.
To understand how the porous graphene helps, first you need to know how today’s lithium-ion batteries work. Like all batteries, lithium-ion cells contain a positive electrode (cathode) and a negative electrode (anode) separated by a chemical medium called an electrolyte and a semi-permeable barrier called a separator.
RELATED: Fern-Like Sheets of Graphene Could Boost Solar Panel Efficiency
When the battery is charged, lithium ions flow to the anode, which is made of graphite. The lithium ions stick to the surface of the graphite and also bury themselves deep in its layers, which is how the energy is stored. When the battery goes to work powering a device, the ions flow from the anode to the cathode, passing through the separator at a steady rate. At the same time, electrons are released at the anode, flow out into the external circuit, and eventually return to the cathode.
To recap, there are two processes that make batteries work, the transport and storage of ions between electrodes, and the release of electrons into the external circuit. To build a battery that stores more energy and recharges faster, you need to optimize the flow of both ions and electrons.
That’s where nanomaterials come in.
 Nanomaterials are named for their impossibly small dimensions, measured in nanometers (one millionth of a millimeter). A number of nanoscale materials have been explored as potential electrode materials that could promise far higher performance than today’s batteries. However, those extraordinary results have only been achieved in the lab using research devices with ultrathin electrodes, not the thicker electrodes required for real-world devices.
Nanomaterials are named for their impossibly small dimensions, measured in nanometers (one millionth of a millimeter). A number of nanoscale materials have been explored as potential electrode materials that could promise far higher performance than today’s batteries. However, those extraordinary results have only been achieved in the lab using research devices with ultrathin electrodes, not the thicker electrodes required for real-world devices.
Graphene is a nanomaterial with some very unique properties. A single sheet of graphene is only one atom thick and consists of a 2D lattice of tightly bonded carbon atoms. Its structure makes it one of the best conductors of electricity on the planet. So if you incorporate graphene into a battery, you can greatly speed up the flow of electrons.
The problem with graphene is that while it’s terrific at moving electrons, it’s impenetrable to ions. If you tried to make an electrode purely out of graphene, the charge/discharge rate of the battery would be slowed by ions having to take detours around the broken edges of the graphene. That’s why researchers decided to punch holes straight through the graphene. 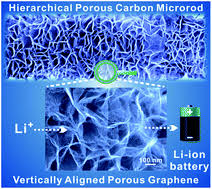
Xiangfeng Duan from the UCLA, one of the authors of the Science paper, explained that the “holey” graphene is used as a conductive scaffold to speed the flow of electrons and direct the transport of ions with maximum efficiency. The graphene scaffold has a three-dimensional “hierarchical” structure with large holes feeding into smaller holes, ensuring that ions are funneled to every available nanometer of the electrode.
“It’s like a transportation network in a city,” said Duan. “You start with wide highways and then you move to narrow local roads to access every home. In the battery, the scaffold allows for the efficient transport of ions across a porous network to directly deliver charge to all of the electrode material.”
RELATED: Seaweed Could Provide a Powerful Boost to Next-Gen Batteries
In their experiments, Duan and his team placed the graphene as a conductive scaffold on niobia (Nb2O5) nanoparticles, a material known for its fast charge/discharge rate. Other labs have experimented with building electrodes solely from materials like niobia in super-thin sheets weighing almost nothing. But Duan said that the performance of the active material in such tiny amounts is canceled out by the bulkier inactive components of an electrode, like the current collectors. In other words, what works in the lab won’t cut it in real-world devices.
By loading the niobia on a graphene scaffold, Duan and his team achieved performance results that were several times greater than with a thin nanomaterial alone. Duan pointed out that the same porous scaffold design they used with niobia could be used with other active materials like silicon or tin oxide, which boast high energy density, the ability to store lots of ions for longer-lasting batteries.
It will still be a while before we see “holey” graphene batteries in real-world devices, said Duan, who calls this paper “a critical step, but just a starting point toward commercialization.” Looking ahead, he could easily see niobia-based batteries that charge up to five or 10 times faster than today’s lithium-ion cells. And batteries made with energy-dense materials like silicon could power laptops for 20 or 30 hours on a single charge, and triple the driving range of an electric vehicle.
“I think this really gives us a pathway toward using these high-performance materials in real-world devices,” Duan said.

[…] […]
LikeLike